Medical Device Accelerated Aging: Everything You Need to Know
Article Summary
Aging studies investigate how a product behaves under normal storage or usage over long periods of time. These studies are generally performed using elevated, stable temperatures. Manufacturers can shorten the time it takes to bring a device into market by quickly determining shelf or storage life, compared to real-time aging.Article Contents
What is Medical Device accelerated aging?
Aging refers to the process by which properties of a product change over time. Aging studies are used to determine how a product behaves under normal storage/use conditions over long periods of time: by subjecting the product to levels of stress that exaggerate normal conditions, the changes can be observed over a shorter timeframe.
Accelerated aging tests involve subjecting samples to elevated temperatures in order to speed up the normal aging process of the samples and simulate real-time aging. This is done to estimate the lifespan of a product, allowing manufacturers to provide an accurate estimation of shelf-life when actual lifespan data is unavailable.
If expiration dates of products are what manufacturers want to establish, then real-time aging studies are necessary and accelerated aging is not sufficient on its own. Real-time aging testing is completed under normal storage conditions rather than elevated conditions.
Why is Medical Device accelerated aging important?
Medical devices need to be able to have long shelf/storage life without it leading to a deterioration in device performance in terms of safety and efficacy. If the devices are stored in sterile packaging, the loss of that sterile barrier system over time can also lead to deterioration/degradation of certain properties of the medical device materials and adhesive components. Similarly, disinfectant products also require stability and shelf-life assessments prior to being put on the market, where the stability of chemical, physical and antimicrobial properties are evaluated after putting the product through aging studies.
Accelerated aging testing is hugely advantageous for medical device manufacturers as it allows them to bring their products into market sooner without compromising on the much-needed safety and stability data of their products.
The FDA report written by GS Clark “Shelf Life of Medical Devices” (April, 1991) provides information and guidance on FDA regulations relating to shelf life of medical devices, the different parameters that are used to determine shelf life, and the processes by which shelf life can be determined.

How is Medical Device aging study performed?
The technique many accelerated aging studies adopt is based on the assumption that the chemical reactions involved in the deterioration and degradation of materials follow the Arrhenius’ reaction rate law. In simple terms, this law states that the aging rate will double with every 10 °C increase in temperature.
The ASTM F1980 standard (“Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices”) is one that is used a lot when creating an aging study. The experimental parameters for this testing include:
- The number of samples/products used in testing
- The ambient storage temperature of the product – typically between 20-25 °C
- The accelerated aging temperature desired to be applied – typically below 60 °C as higher temperatures could cause non-linear changes meaning the Arrhenius law cannot be applied.
- Accelerated aging factor (AAF), which can be calculated using the following equation:
Where:
TAA = accelerated aging temperature (°C)
TRT = ambient temperature (°C)
Q10 = rate of chemical reaction, typically 2
- Humidity considerations must also be added in the aging process as the standard states: “Since sterile barrier systems and medical device are stored in environments that comprise varying levels of ambient humidity, and since the properties of some materials may depend on the level of absorbed moisture […] it is important to consider not only the accelerated aging temperature conditions but also the ambient relative humidity during that accelerated aging.”

Chemical disinfectant aging studies
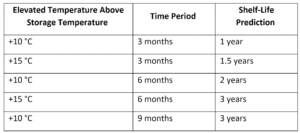
For chemical disinfectants, the Therapeutic Goods Administration (TGA) of the Australian Government provide instructions on assessing the chemical stability and shelf-life of disinfectants. In this test, the disinfectant is isolated in a humidity chamber with elevated test temperature and tested for stability at the initial time point as well as at 3 other time points during the period of accelerated aging. The table below demonstrates the shelf-life prediction within these time intervals, as per the TGA guidelines:
The physical, chemical and antimicrobial properties of the disinfectant are assessed during the aging study:
- Physical properties such as appearance, pH, boiling point, odour, and material compatibility are common characteristics that are assessed.
- The 3 key points to be considered in chemical stability, according to the TGA, are:
-
- Determining the active concentration of active substances with an appropriate detection assay, e.g. Fourier-Transform Infrared Spectroscopy (FTIR), or Gas Chromatography (GC).
- Active concentration of active substance should not fall below 90% of the initial label claim at the end of shelf-life analysis.
- All chemical test analysis should run in duplicate samples.
- Antimicrobial stability is assessed via efficacy studies of which there are many international methods such as EN 13727, EN 13624, EN 16615, EN 13697, etc.
Get It Done, With Certainty.
Contact us about your accelerated and real-time aging study requirements, we aim to respond the same day.
Get resources & industry updates direct to your inbox
We’ll email you 1-2 times a week at the maximum and never share your information