Securing Innovation: Strategic Intellectual Property (IP) Layering for Medical Devices
Article Summary
Medical device innovation cannot rely on a single patent for protection. By layering multiple IP rights - covering core technologies, components, and applications - companies can secure stronger protection, extend market exclusivity, and deter competitors. Case studies of PureFize and Oxitone show how diversified IP portfolios safeguard R&D investments while boosting commercial and competitive value.Article Contents
What Makes IP Strategy Essential for MedTech Growth?
The field of medical devices is vast and ever-evolving. Ranging from simple items like thermometers and surgical instruments to complex technologies such as pacemakers, diagnostic imaging systems, and robotic surgical tools, medical devices come in many forms and levels of sophistication. They are essential tools in modern healthcare and play a critical role in the diagnosis, treatment, monitoring, and prevention of diseases and medical conditions.
Intellectual property (IP) plays a vital role in the medical device industry. Protecting innovations not only encourages investment and research but also helps companies maintain a competitive edge. This article explores how a multi-faceted IP approach can enhance legal protection, increase commercial value, and strengthen competitive advantage in the context of medical devices. Two real-world examples of medical devices are used as case studies to explore layering of IP.
What Is Intellectual Property (IP)?
IP refers to creations of the mind such as inventions, literary works, artistic works, and designs. There are a number of ways to protect IP, depending on the type and nature of IP itself. For example, design rights protect the way a product looks. Copyright protects original works of authorship, such as books, music, and software. Trade marks can be words, phrases, symbols, designs, or a combination thereof which are used to distinguish the goods and services of one party from those of others.
Perhaps the most complex form of IP protection is patent rights. Patents provide a patent holder with negative monopoly rights, i.e. they grant the patent holder the right to exclude others from making, using, or selling the invention for a limited period of time, in return for publicly disclosing the invention.
Why Is a Single Patent Not Always Enough for Robust Protection?
It is a common misconception that a single patent can fully protect a medical device, covering all its components and functionalities. In reality, medical devices are typically safeguarded by a portfolio of patents, each targeting specific aspects, such as mechanical features, materials, or methods of use. This layered approach provides more comprehensive protection and helps mitigate the risk of design around strategies by competitors.
What Role Do IP Rights Play in Medical Devices?
How Can IP Layering Protect UVC Device Innovation?
UVC (Ultraviolet C) light is a form of ultraviolet light with wavelengths between 200-280 nm, known for its ability to kill or inactivate microorganisms like bacteria, viruses, and fungi. UVC light disrupts the DNA or RNA of microorganisms, preventing them from reproducing and causing infection. UVC disinfection offers a chemical-free alternative to traditional disinfectants.
There are three typical types of UVC Devices:
1. UVC lamps: Used for disinfecting air and surfaces in large spaces.
2. Portable UVC wands: Handheld devices for quick surface disinfection.
3. UVC sterilisation chambers: Enclosed devices that disinfect objects.
The pandemic heightened awareness of hygiene and disinfection, increasing demand for UVC disinfection devices for both air and surface sanitisation. UVC disinfection is now used in healthcare settings, commercial spaces, public transportation, and even personal items like mobile phones and face masks. The growing demand for such devices has boosted research and innovation in this field, increasing with it need for and importance of protecting associated IP.
PureFize Technologies AB are a Swedish research and technology company focused on chemical-free disinfection and hygiene improvement using UV technology. It’s PureFize® product platform is a modular and scalable UV disinfection solution with applications in numerous industries.
Different aspects of its platform devices are protected by patent rights. For example, they have 3 granted European patents and 5 European patent applications (which are in progress, but not yet granted) and a patent application in the international phase (a very early stage). Their patent portfolio includes protection for a device (WO2025005850), for a portable device (EP4466033), for a means of disinfecting fluid (EP3475229), for a means of disinfecting surfaces (EP4061431), for a method of manufacturing the UV lamps (EP3465290), and for the systems behind the working of the devices (EP3649669, EP3586351, EP3552225 and EP3356864). Below are images from two of PureFize’s patent rights which demonstrate the variety of medical devices that can be created from the same platform technologies.
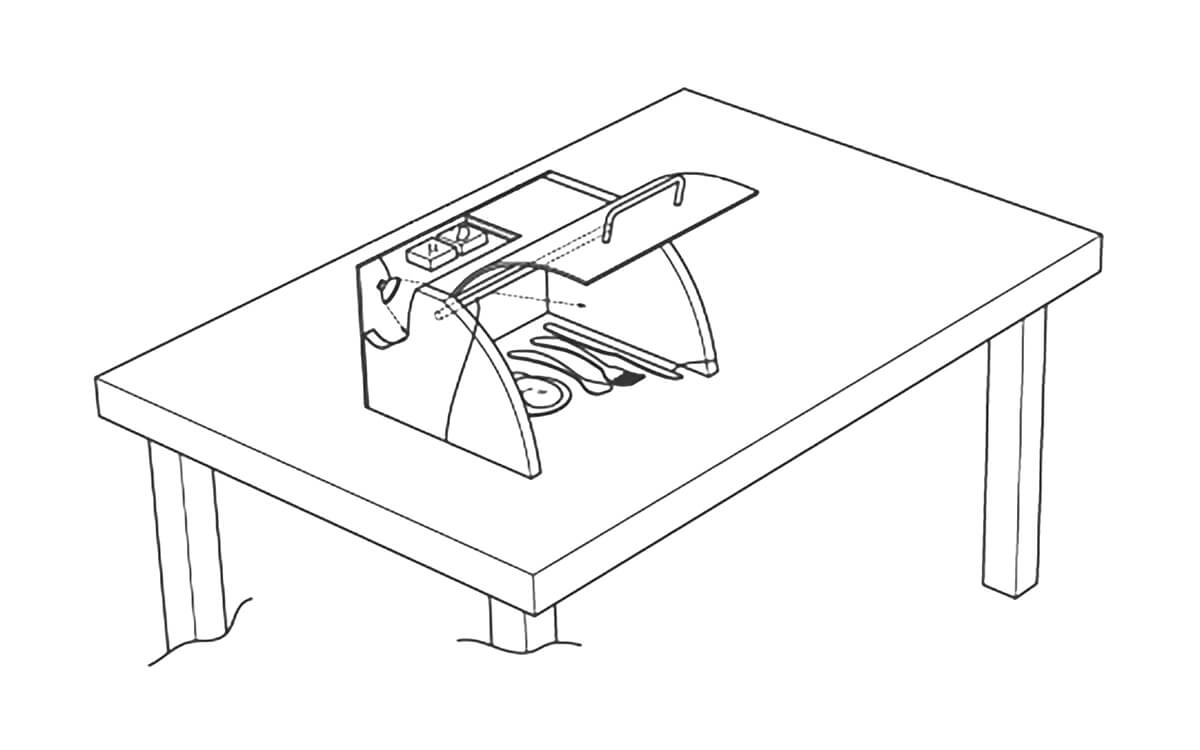
Image of a portable UVC disinfection device from European patent application EP4466033
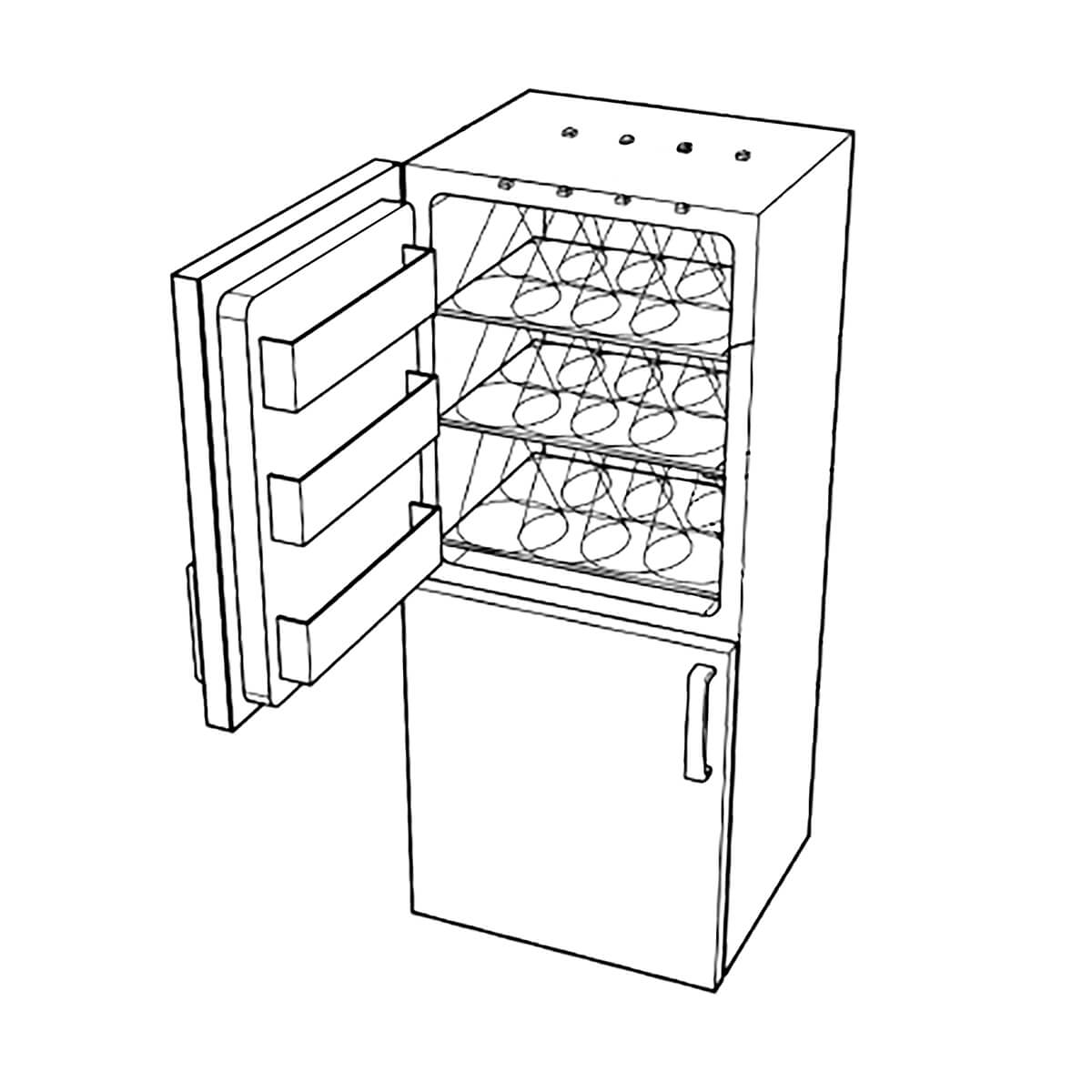
Image of a UVC lighting array for disinfecting surfaces from European patent application EP4061431
PureFize’s patent portfolio highlights how medical devices can be protected through multiple patents, each covering a specific feature, component or aspect. These protections can be layered, meaning a single product may incorporate several patented inventions. This approach not only enhances legal protection but also adds commercial value and strengthens the company’s competitive position in the med-tech market by creating a dense and evolving portfolio of patent rights that is difficult for competitors to navigate or design around. In addition, by layering patents over time to cover improvements, variations, or new applications, companies can extend the overall duration of protection and maintain exclusivity beyond the life of any single patent.
How Do Wearable Oxygen Monitors Use IP to Compete?
Wearable technology has significantly advanced in recent years, particularly in the healthcare sector. A notable innovation is wearable oxygen monitoring devices. These compact, non-invasive gadgets are designed to continuously monitor a person’s oxygen saturation (SpO2) levels in real-time. Oxygen saturation is a critical indicator of how effectively oxygen is being transported throughout the body and wearable oxygen monitors provide individuals with the ability to track their oxygen levels conveniently. Such devices have increased in popularity across numerous markets including healthcare, sports and fitness, wellness and lifestyle, and they even have military applications.
Oxitone is a relatively young US-based company that has developed a wearable device for continuous oxygen saturation monitoring. Unlike traditional pulse oximeters that require fingertip or earlobe sensors, Oxitone’s wearable device can track oxygen levels using a non-invasive wrist-worn sensor. Its oxygen monitoring system employs advanced optical sensors and algorithms to continuously measure blood oxygen levels, heart rate, and other vitals. The key innovation lies in the way the device fits and measures the user’s physiological data non-invasively on the wrist, overcoming the limitations of traditional methods that require more direct contact with the body.
Oxitone’s patent portfolio encompasses different products utilising it’s technology, for example for a wearable sensor (EP2750604), a wrist sensor (EP3558096), a wearable device specifically for foetuses (EP4615323) and a general SpO2 determination device (WO2011013132). Below is an image of a wearable wrist sensor from one of Oxitone’s patents.
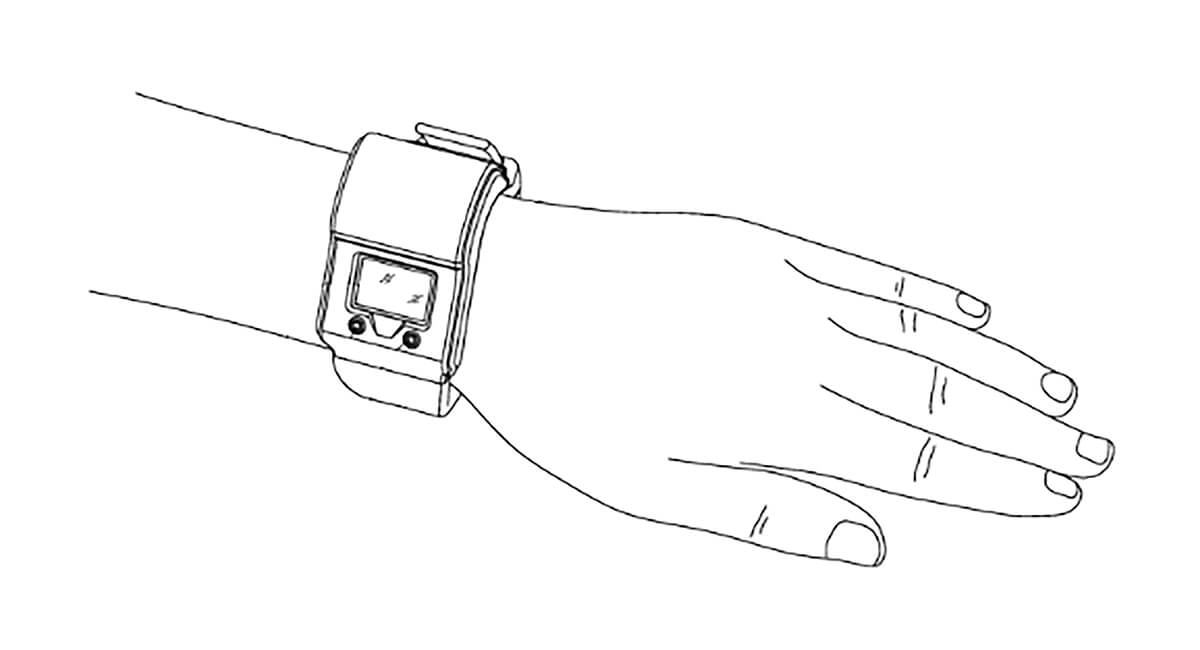
Image of a wrist sensor from European patent application EP2750604
How Does IP Shape Innovation in Medical Devices?
In the medical device industry, patents play a crucial role in safeguarding innovation. As demonstrated by the examples of PureFize and Oxitone, a single medical device or technology can be protected through multiple patents, each covering distinct components, features or aspects. Rather than relying on a single broad patent, the companies have built diversified portfolios that protect both core technologies and specific applications. This not only helps safeguard their R&D investments but also creates flexibility in product development and licensing. Ultimately, patents can work together to provide comprehensive protection which enables sustained innovation and growth in the dynamic med-tech space.
References
https://www.oxitone.com/
https://www.purefize.com/
https://register.epo.org/smartSearch?searchMode=smart&query=purefize
https://register.epo.org/application?number=EP18829022
https://register.epo.org/application?number=EP16849106
https://register.epo.org/application?number=EP24832575
https://register.epo.org/application?number=EP23743579
https://register.epo.org/application?number=EP20888995
https://register.epo.org/application?number=EP18753746
https://register.epo.org/application?number=EP17878779
https://register.epo.org/application?number=EP17815816
https://register.epo.org/application?number=EP17803169
https://www.tmdn.org/tmdsview-web/#/dsview/results?page=1&pageSize=20&criteria=W&applicantName=PureFize%20Technologies%20AB
https://www.tmdn.org/tmview/#/tmview/results?page=1&pageSize=30&criteria=C&appName=PureFize%20Technologies%20AB
https://register.epo.org/smartSearch?searchMode=smart&query=oxitone
https://register.epo.org/application?number=EP23888225
https://register.epo.org/application?number=EP17829296
https://register.epo.org/application?number=EP12827666
https://register.epo.org/application?number=EP10804005
https://www.tmdn.org/tmdsview-web/#/dsview/results?page=1&pageSize=20&criteria=W&applicantName=Oxitone%20Medical%20Ltd.
https://www.tmdn.org/tmview/#/tmview/results?page=1&pageSize=30&criteria=C&appName=Oxitone%20Medical%20Ltd.
Disclaimer. The views and opinions expressed in this article are solely those of the author and do not necessarily reflect the official policy or position of Test Labs Limited. The content provided is for informational purposes only and is not intended to constitute legal or professional advice. Test Labs assumes no responsibility for any errors or omissions in the content of this article, nor for any actions taken in reliance thereon.
Get It Done, With Certainty.
Contact us about your testing requirements, we aim to respond the same day.
Get resources & industry updates direct to your inbox
We’ll email you 1-2 times a week at the maximum and never share your information
