What is the health technical memorandum?
Health Technical Memorandum (HTM) 01-01 consists of a list of documents (part A-E) that provide guidance and information on the decontamination and reprocessing of reusable surgical instruments used in healthcare.
Parts A and B have been summarised and discussed in Test Labs’ previous post. In this post parts C to E will be covered, which go into detail about the cleaning and sterilisation of reusable medical devices.
HTM 01 01 Part C: Steam sterilisation
The standards and guidance on steam sterilisation are detailed in Part C, where the considerations found in part B are applied specifically to steam sterilisers. Outlined are considerations for the design and pre-purchasing, the validation and verification, as well as the operational management of steam sterilisers. This HTM only considers clinical sterilisers and is not applicable for laboratory sterilisers. Part C covers sterilisers that use high-temperature steam, whereas Part E covers other processes including low-temperature processes.
High-temperature steam sterilant is preferred, and machines using other sterilants should be used only in cases where high temperature would cause damage to the load or not sterilise the load. Sterilisers should only be used for the load that they are designed for. The Authorising Engineer (Decontamination) (AE(D)) can provide guidance on the modification of operating cycles to suit particular loads. The ‘Particular Specification for porous load sterilizers’ attached at the end of HTM 01-01 Part C can be used to compare technical and financial data provided by the tenderer to aid the purchaser in confirming whether the steriliser is acceptable for the intended use, as well as enabling a like-for-like tender analysis to be made. Additionally, BS EN 285 (“Sterilization – Steam sterilizers – Large sterilizers”) and HTM 01-01 Part B should be referred to when preparing a procurement specification for a steriliser.
Porous load sterilisers should conform to specifications in BS EN 285 and safety specifications in BS EN 61010-2-040 (“Safety requirements for electrical equipment for measurement, control, and laboratory use. Particular requirements for sterilizers and washer-disinfectors used to treat medical materials”). They are different to other high-temperature sterilisers by the following features:
- They trap both air and moisture, therefore contain a vacuum system to ensure enough air is removed from the chamber before steam is admitted. The vacuum system also helps to ensure the load is adequately dry at the end of the cycle by reducing the pressure during the drying stage of the cycle.
- They contain an air detector within the chamber to ensure sufficient air has been removed from the chamber before the ‘plateau period’ starts.
- They contain a heated jacket to prevent condensation occurring on the chamber walls and to assist drying of the load.
Sterilisation process validation
Sterilisation processes should be validated before use, the process performance should be routinely monitored, and the sterilisation equipment should be maintained as per manufacturer’s instructions. Procedures to be completed by manufacturers to confirm acceptable performance are outlined in BS EN 285. After installation of the steriliser, Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ) procedures are to be performed as defined in BS EN ISO 17665 (“Sterilization of health care products — Moist heat”).
IQ tests involve checks on the ancillary equipment and the engineering services, as well as preliminary and functional checks on the sterilisers. In addition to these checks, BS EN 285 requires manufacturers to perform a sound power test to measure the total sound power radiated from the machine. A contractor should perform the IQ tests before OQ tests are done. PQ tests should only be done once IQ and OQ tests have been satisfactorily completed. PQ is done to obtain evidence that when the steriliser is used as per the process specification and in accordance with the pre-defined acceptance criteria, the results are consistently the same. It allows you to establish the expected performance for any given cycle and loading conditions, which can then be used as a benchmark to compare subsequent cycles against.
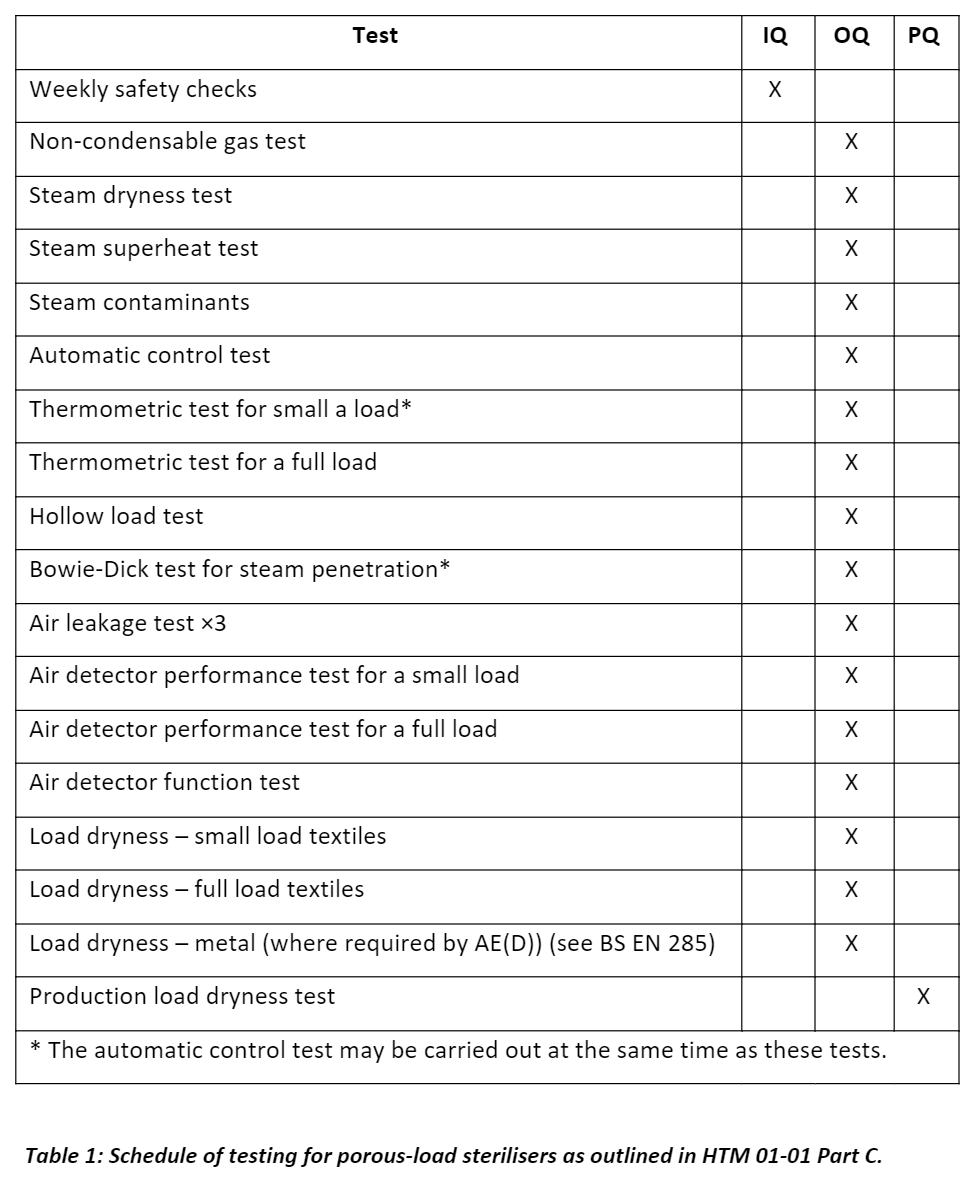
Table 1 below shows the schedule for validation tests to be completed for porous load-sterilisers:
Chemical quality of steam
The quality of steam in terms of dryness, superheat, and level of non-condensable gases is also required to be tested and examined after installation and before being handed over to the user. BS EN ISO 17665 provides the requirements for the environment in contact with a medical device and BS EN 285 provides guidance on the chemical quality of steam. HTM 01-01 Part C outlines the importance of steam quality due to concerns about harmful effects of contaminants in the steam on patients. Contaminants known to have adverse effects on patients are:
- metals (e.g., cadmium, lead, mercury and other heavy metals),
- organic compounds, primarily filming amines and other chemicals used in boiler treatment,
- microorganisms,
- pyrogens (bacterial endotoxins),
- particulate matter.
Contaminants that are of concern for their damaging effects on materials of load items and the steriliser are:
- alkaline earth metals (e.g., calcium and magnesium), which can lead to the build-up of limescale,
- iron, which is corrosive to stainless steel,
- chlorides, which also contribute to corrosion in the presence of oxygen,
- phosphates and silicates.
HTM 01 01 Part D: Washer-disinfectors
In the same way that Part C details the standards and guidance applicable to steam sterilisers, Part D provides the same information but relevant to washer-disinfectors. A washer-disinfector (WD) is used to clean and disinfect reusable items. The washer-disinfector process involves 2 stages: cleaning and microbial inactivation (see figure 1).
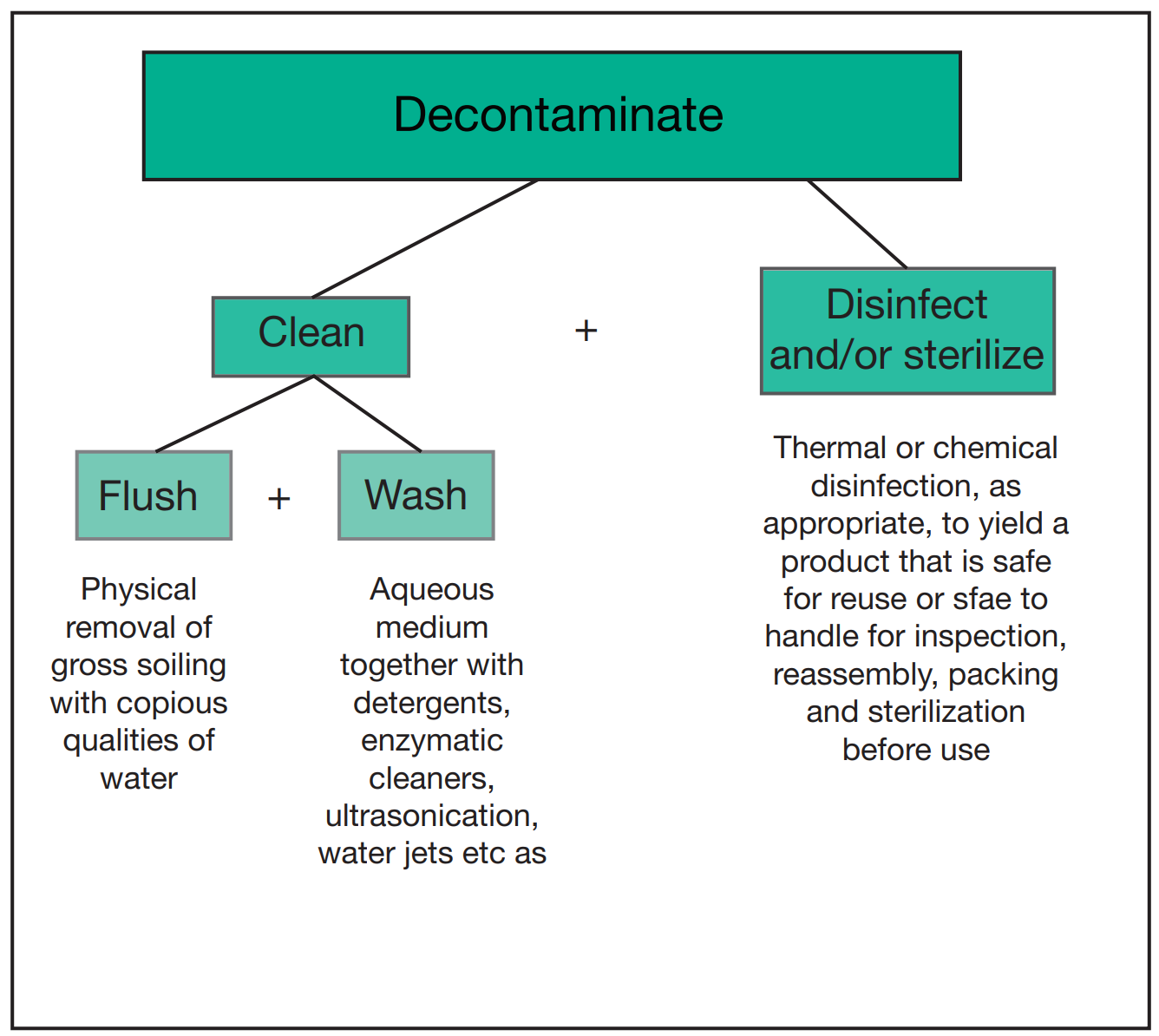
Figure 1: “Elements of the decontamination process”. Image obtained from HTM 01-01 Part D
Source: https://www.england.nhs.uk/wp-content/uploads/2021/05/HTM0101PartD.pdf
The choice of WD to be purchased is dependent on the load it would be used for. HTM 01-01 Part D provides a ‘Particular specification for washer-disinfectors used for reprocessing surgical instruments’ that can be used in the pre-purchasing process and for tender analysis/comparisons, and BS EN ISO 15883 Parts 1 and 2 provide further guidance on washer-disinfector specifications and compliance. Specifications detailed in Part D include the WD construction materials (should comply with BS EN ISO 15883), the integral air compressors, calorifiers and tanks (should comply with BS 853-1), the dosing systems, how they should be cleaned, the drainage, and many other factors.
When compressed air is used and in contact with the load items, it must be ‘medical grade’ compressed air, i.e., free from oil and particulates. If chemical additives are used to enhance the disinfectant process, which is not necessary for all applications, there needs to be a rinsing process that removes the chemical additives. Compatibility testing is required for chemical additives used to determine whether the chemicals are compatible with the following:
- the WD construction materials
- the process being operated
- the water quality available
- the items to be processed and their intended use
- any decontamination process (e.g. sterilisation) that would follow
Ultrasonic cleaners
Ultrasonic cleaners are specifically mentioned for use with instruments of high–grade steel and delicate microsurgery instruments and dental instruments that have deep interstices that may be contaminated with body tissue (e.g., reamers, drills, and burrs). They work by exposing the item to high frequency sound waves in a liquid cleaning medium, agitating the liquid and causing cavitation. Cavitation is the formation and collapse of small cavities due to pressure difference in a liquid. This process provides a deep cleaning effect. Safety specifications for ultrasonic cleaners are included in BS EN 61010-1. The ultrasonic frequency used should be in the range 35 kHz ± 5 kHz, and the energy input used may range from 5 WL-1 to 20 WL-1. Ultrasonic cleaners must undergo validation and periodic tests whereby the activity is measured by seeing the erosion pattern created on aluminium foil that would be exposed in the ultrasonic bath for a short period. The period of exposure will depend on the foil thickness and hardness, as well as the operating frequency, the watt density and the temperature of the bath. Tests performed during commissioning are to be used to establish the variation in activity at different positions and levels within the bath, and to determine the erosion pattern that will occur. For more detailed information on how to perform the test, see HTM 01-01 Part D.
Cleaning and disinfection validation
The validation of the cleaning and subsequent disinfection processes needs to be validated to show that all types of loads that are used with these processes are consistently cleaned and disinfected to the required level. The level of residual contamination and adequacy of cleaning is usually assessed by visual inspection, however in some cases this is not sufficient, e.g., the internal surfaces of instruments with lumens cannot be checked visually for any soiling that has remained or any residual chemical additives left from the WD. Cleaning and disinfection processes need to be validated before use, the process performance should be monitored during routine use, the calibration of WD controls and instrumentation should be verified, and there should be a suitable maintenance programme in place for the equipment.
HTM 01-01 Part D outlines recommended control protocols, which include tests and checks carried out during manufacturing, after delivery, during validation and periodically thereafter. This all ensures the WD is fit for its intended use. Type tests are conducted by the manufacturer at the factory to demonstrate that the WD design complies with BS EN 15883 Parts 1 and 2 by performing the test on a representative sample. Similarly, work tests are also conducted at the factory by the manufacturer, but these tests ensure each WD meets the specification. Installation and operational tests are carried out by the manufacturer on site and include checks on the ancillary equipment and functional checks on the WD. Testing protocols for WDs, outlined in HTM 01-01 Part D and detailed in BS EN 15883 Parts 1 and 2, are shown in Table 2:
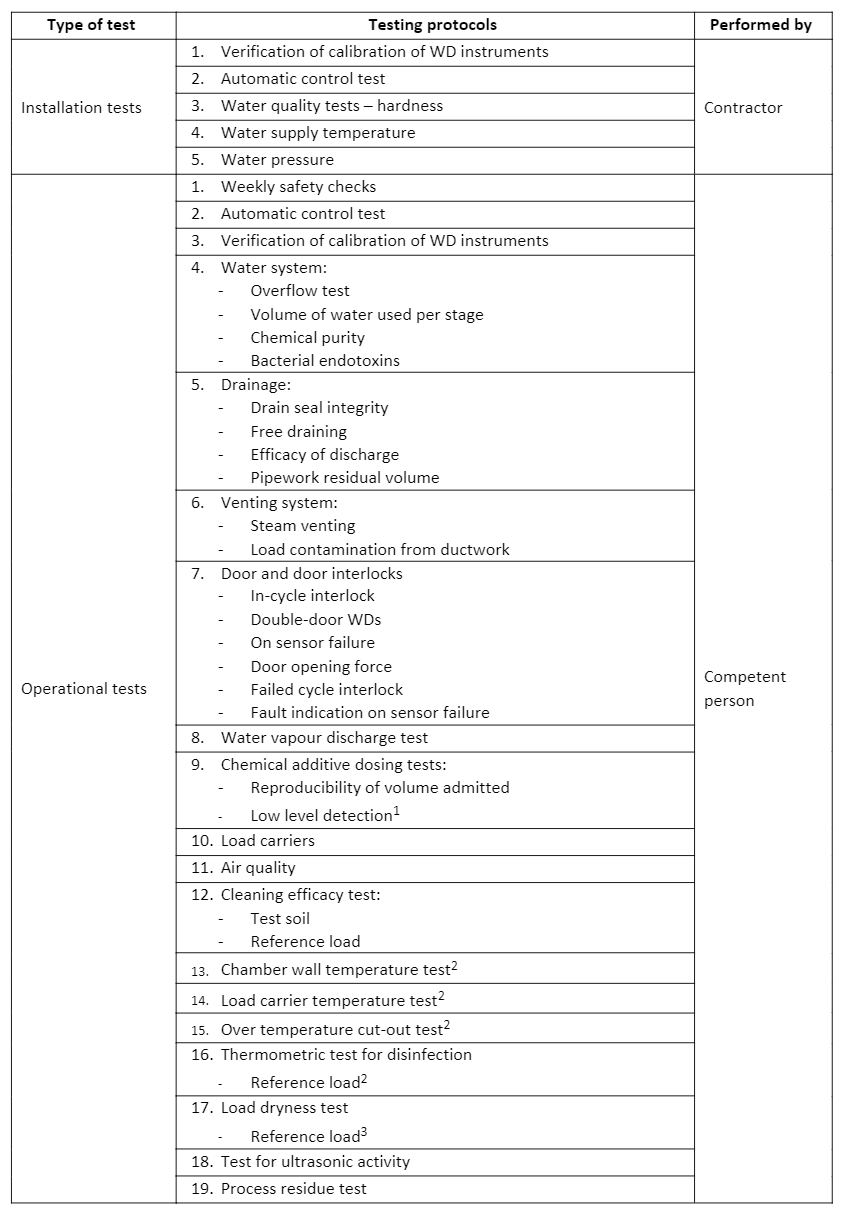
1 Only required for WDs with automatic chemical dosing
2 Only required for WDs with a thermal disinfection stage
3 Only required for with a drying stage
Performance Qualification (PQ) procedures
For PQ tests surrogate devices are used, which are test pieces that have the same characteristics as the device and allow for the cleaning and disinfecting processes to be appropriately monitored. The surrogate device must be constructed of the same materials and have the same surface finishes as the emulated device. The geometry and thermal mass of the surrogate device should also be as close as practically possible to the device. PQ tests on WDs include (but aren’t limited to) cleaning/disinfection efficacy tests, the detection of process residues, thermometric tests, load dryness tests, and automatic control tests. HTM 01-01 Part D provides guidance on how these different tests should be performed, who is responsible for performing them, the results that should be expected, and how regularly they should be performed.
The water supply used with the WD must also be in accordance with relevant by-laws to prevent waste, excessive consumption, misuse or contamination (see the Water Supply (Water Fittings) Regulations 1999). The quality of the water supplied to the WD at all stages in the decontamination process is critical to ensuring the process performs successfully. WDs can be supplied with both hot and cold water, and it is recommended that if a process requires hot water, it is better to supply hot water directly to the WD rather than heat cold water to temperature within the WD cycle process. Water quality should be compatible with the following at each stage of the process:
- WD construction materials
- Load items to be processed
- Chemical additives (if used)
- Process requirements of that stage
The key factors of the water quality to consider and monitor are detailed in HTM 01-01 Part D, but are the following:
- Hardness
- Temperature
- Ionic contaminants (e.g., heavy metals, halides, phosphate and silicates)
- Microbial population
- Bacterial endotoxins
Water treatment is needed to ensure optimum quality of water used in each stage of the WD process cycle. Different types of water have qualities to be applied in different ways, such as cold water for flushing, potable water for flushing/washing, deionised water for final rinse, etc. There are generally 3 methods of water treatment used in WDs during the process cycles:
- Water softeners where calcium and magnesium ions in the water are replaced with sodium ions. Base-exchange softeners can lead to a significant increase in microbial population present in the water, so this needs to be considered when deciding which stage of the cycle the softeners are used with certain products/load items.
- Water deionisers that remove most of the dissolved ionic material by ion-exchange. This should not be used in the final rinse for products/load items used in invasive procedures without further decontamination processing by heating/filtration etc., because deionised water can become contaminated with microorganisms.
- Reverse osmosis which removes almost all dissolved inorganic contaminants, as well as a lot of organic material, bacterial endotoxins and microorganisms.
HTM 01-01 Part D provides guidelines and details for water system tests conducted on water samples obtained from draw-off points within the system. These tests are analytical methods to determine the biological, physical and chemical properties of water samples and include temperature, pressure and pH tests. Further guidance of which tests are appropriate can be found in BS 1427:2009 (“Guide to on-site test methods for analysis of water”).
HTM 01 01 Part E: Alternatives to steam for the sterilisation of reusable medical devices
Although the majority of medical devices can be used in steam sterilisers, for some specialist instrumentation and devices it is not appropriate to reprocess using steam sterilisation as the devices can be damaged under steam at high temperatures. HTM 01-01 Part E provides guidance for commissioners and regulators of non-steam sterilisers, as well as outlining the role of non-steam sterilisation techniques, the quality and safety standards of non-steam sterilisation, and the medical device/instrument compatibility.
Devices that cannot undergo steam sterilisation are known as thermolabile medical devices and for the reprocessing of such devices, non-steam sterilisation techniques are required. Furthermore, it is mentioned that the use of non-steam sterilisation may provide more significant inactivation of prions. The use of low temperature electronic gas plasmas is a potential technique that may be used in the future for non-steam sterilisation, and the current technologies used include:
- Ethylene oxide
- Gaseous hydrogen peroxide
- Ozone
Since no specific quality framework for alternative sterilisation methods exists within European Norms (EN) or BSI standards, policy guidance for non-steam sterilisers will follow the broad outline of EN ISO 14937:2009 (“Sterilization of health care products”) and be adapted to the specific technology utilised.
If you have any questions or need further assistance in understanding and implementing these requirements for your reusable medical devices, please don’t hesitate to get in touch with us. Our team is ready to discuss your specific requirements and provide guidance tailored to your needs.