FDA Requirements for Reusable Medical Device Validation
The FDA requires manufacturers of certain reusable medical devices to include validated instructions for use and validation data on cleaning, disinfection, and sterilization in their 510(k) premarket notifications. In this blog post, we provide guidance on the requirements and criteria for validation data related to instructions for use, in accordance with 21 CFR 801.5. For more information on premarket registration and classification requirements, refer to “FDA Approval for Medical Devices: Everything You Need to Know“
Cleaning validation steps
According to the FDA, effective cleaning should:
“minimize the soil transfer from one patient to another or between uses in a single patient; prevent accumulation of residual soil throughout the product’s use life”;
According to ANSI/AAMI ST98:2022
- Test soil choice and test soil validation:
ASTM F3293-18 offers guidance for an artificial test soil, the artificial test soil chosen should allow at least two clinically relevant soil components to be quantified for validation testing (e.g., total organic carbon, protein).
- Inoculation and conditioning:
Soil inoculations should mimic worst-case clinical use conditions. All locations on the medical device likely to contact patient materials should be soiled, including all locations that are difficult to clean.
- Cleaning of the medical device according to the IFU:
The personnel responsible for the IFU validation would then clean the soiled medical device according to the IFU with no deviation.
- Extraction of residual test soil (analytes):
Devices should be subjected to a validated method of extraction for recovery of residual soil. The extraction method should be completely described for each device and its recovery efficiency should be determined as part of its validation.
Cleaning validation criteria
The following acceptance levels must be met in order to validate the cleaning instructions provided by the manufacturer:

A minimum of 2 analytes must be measured for the medical device being tested regardless of standard.
Cleaning validation standards
- AAMI TIR30 – A Compendium of Processes, Materials, Test Methods, and Acceptance Criteria for Cleaning Reusable Medical Devices.
- ANSI/AAMI ST98:2022 – Cleaning Validation of Health Care Products – Requirements for Development and Validation of a Cleaning Process for Medical Devices.
- ISO 15883-5:2021 – Washer-disinfectors — Part 5: Performance Requirements and Test Method Criteria for Demonstrating Cleaning Efficacy.
- ASTM F3293-18 – Standard Guide for Application of Test Soils for The Validation of Cleaning Methods For Reusable Medical Devices.
Sterilisation & Disinfection validation steps
Following cleaning, medical devices that are not returned into service will need to go through additional antimicrobial steps such as sterilisation and disinfection.
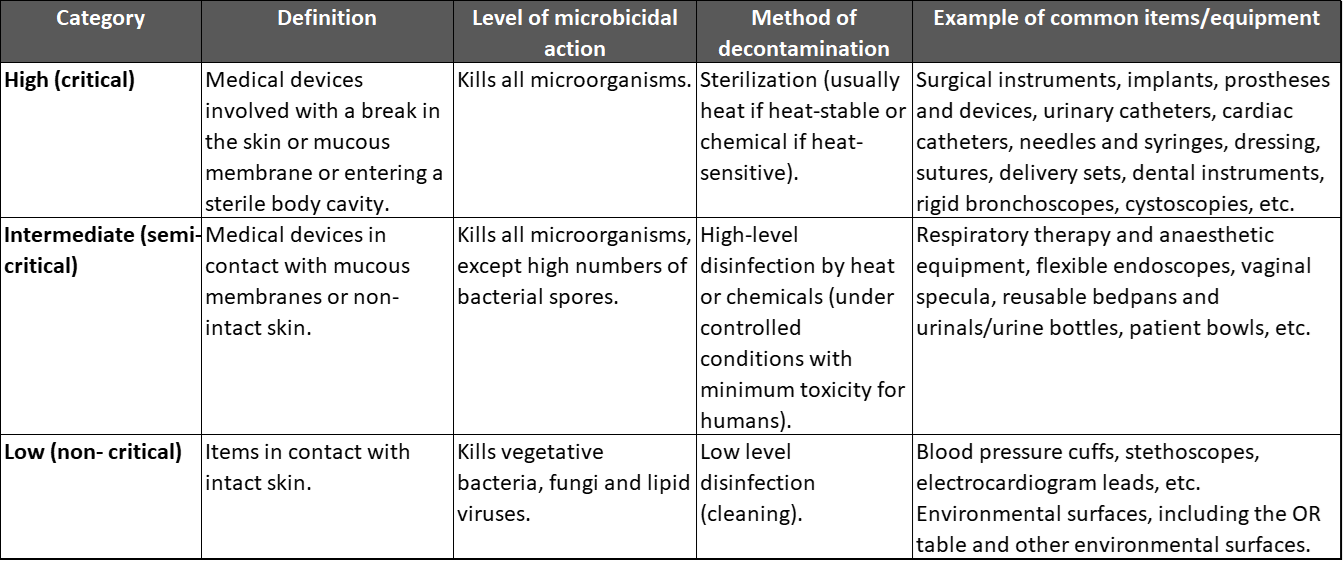
CDC considers the Spaulding Classification to offer rational guidance on the extent to which a medical device should be processed relative to criticality.
Spaulding classification
The Spaulding Classification is a categorization scheme for patient-care items and equipment based on the degree of risk for infection involved in their use. The classification, which was developed more than 30 years ago by Earle H. Spaulding, divides instruments and items into three categories: critical, semicritical, and noncritical. This approach has been widely used by infection control professionals to plan methods for disinfection or sterilization, and it is employed in various CDC guidelines for handwashing, hospital environmental control, and infection control in healthcare facilities.
Sterilisation validation at Test Labs ‘Overkill method’
The goal of the overkill method based on EN ISO 17665-1:2006 and ANSI/AAMI/ISO 17665-1:2006, is justification of validity of a sterilisation process by demonstrating its effectiveness at killing a predesignated number of microorganisms.
The validation of an F0 value that gives a SAL of 10-6 (or a value determined to be satisfactory by the medical device manufacturer) should be performed by the overkill method partial cycle approach.
The method aims to justify a SAL of 10-12 as being achievable in real world conditions, using the F0 provided by the medical device manufacturer, if a SAL of 10-6 is achieved at half the provided F0 value.
Sterilisation validation standards
- EN ISO 17665-1:2006 – Requirements for the Development, Validation and Routine Control of a Sterilisation Process for Medical Devices
- ANSI/AAMI/ISO 17665-1:2006 – Sterilization of Health Care Products – Moist Heat – Part 1: Requirements for the Development, Validation, and Routine Control of a Sterilization Process for Medical Devices
Disinfection validation steps
Thermal disinfection validation involves performing temperature mapping on the device being used to disinfect the medical device during the disinfection cycle.
According to ISO 15883-2:2006, during the disinfection cycle, the following requirements must be met:
- The operating cycle specified by the medical device manufacturer shall include a thermal disinfection stage which outlines a temperature that adheres to a minimum A0 of 600* for:
- All surfaces of the load to be disinfected
- All internal surfaces of the chamber and on the load carrier.
- A0 of 600 equivalent to 80 °C for 10 minutes, or 90 °C for 1 minute.
Chemical disinfection validation, based on ANSI/AAMI ST58:2013, medical devices are inoculated in the worst-case location providing the highest challenge to the disinfection process.
The biological indicator e.g., Mycobacterium to prove high level disinfection claims or Staphylococcus aureus for intermediate and low-level claims, is allowed to dry on the medical device.
The medical device is then disinfected according to the IFU, the disinfectant is prepared and applied to the medical device for the required contact time. The biological indicator on the medical device is then extracted, and log reduction testing is performed.
Disinfection validation standards
- ANSI/AAMI ST58:2013 – Chemical Sterilization and High-Level Disinfection in Health Care Facilities.
- ISO 15883-2:2006 – Requirements and Tests for Washer-Disinfectors Employing Thermal Disinfection for Surgical Instruments, Anaesthetic Equipment, Bowls, Dishes, Receivers, Utensils, Glassware, etc.
Adhering to the criteria outlined in the aforementioned standards is essential for ensuring a successful submission of your 510(k) for reusable medical devices. By meeting these requirements and validating the instructions for use, as well as the cleaning, disinfection, and sterilization processes, you can demonstrate compliance with FDA regulations and enhance the safety and effectiveness of your medical devices. Meeting these standards not only facilitates regulatory approval but also instills confidence in healthcare providers and patients regarding the proper use and maintenance of the devices.
If you have any questions or need further assistance in understanding and implementing these requirements for your reusable medical devices, please don’t hesitate to get in touch with us. Our team is ready to discuss your specific requirements and provide guidance tailored to your needs.