EN 17272 – Test Method for Airborne Disinfection
Article Summary
Healthcare-associated infections (HCAIs) pose a major challenge, costing the NHS over £1 billion annually, and driving the need for effective disinfection beyond manual cleaning. EN 17272:2020 provides a standardised method to evaluate automated airborne disinfection systems, ensuring consistency in testing efficacy and distribution. The test measures microbial reduction on surfaces under controlled conditions, helping validate new technologies that support infection prevention in healthcare settings.Article Contents
The importance of the EN 17272 test
Healthcare-associated infections (HCAIs) are one of the biggest threats to the healthcare system. These infections are usually acquired after hospitalisation and manifest 48 hours after admission to the hospital. These infections are monitored closely by agencies such as the National Healthcare Safety Network (NHSN), the National Institute of Health and Care Excellence (NICE), and the Centre for Disease Control and Prevention (CDC).
According to NICE, 300,000 people are diagnosed with HCAI in England every year, costing the NHS an estimated £1 billion a year. This could be due to contamination of equipment and other materials or cross-contamination within the hospital.
Disinfection of healthcare spaces therefore must be routinely implemented. Manual cleaning using chemical disinfectant such as sprays or wipes is still seen as the golden standard despite some limitation mainly due to “human error”.
Over the last few decades automated disinfection technology has been developed to help and support manual cleaning in healthcare settings. Automated disinfection systems work based on the dispersion of the active ingredient (e.g., ozone, hydrogen peroxide etc.) within an enclosed room in the form of vapour, gas or aerosol, without human intervention.
Despite some regulation coming from France, NFT 72-281, there was a lack of standardised methods to evaluate the efficacy of these new technologies.
Overview of the EN 17272:2020 test
When was the EN 17272 test released?
In March 2020 the European disinfectant standard writing body, the European Standardization Committee (or Comité Européen de Normalisation, CEN) announced the release of a new standard that would cover the requirements and methodology for testing efficacy of automated airborne disinfection technology; EN 17272:2020.
How does the EN 17272 test work?
In brief, the airborne surface disinfection system is placed within an enclosed test chamber at a specified distance and location, and the active ingredient (e.g., ozone, hydrogen peroxide etc.) is injected the form of a gas, vapour and or aerosol. The process will be started remotely (with no people present inside the chamber) and it will disinfect the surfaces, and not the air, in the room. Results are expressed in logarithm scale to assess compliance to the standard.
The method consists of two main parts that must be performed for each product and system.
Efficacy Test
Stainless steel carriers are inoculated with a suspension of microorganism and interfering substance (replicating clean or dirty conditions) in triplicate per each organism and left to dry. Two control carriers are prepared similarly and will be left outside the test chamber with similar environmental conditions.
Once dry, they will be moved inside the chamber and clamped vertically at 1-1.5m height facing away from the release source.
The airborne surface disinfection system is then placed within the test chamber at specified distances from the challenge (e.g., for a 65m3 chamber carriers are place 3.6m away from the system) and the disinfection cycle is started.
The chamber must be equilibrated for temperature (as standard 20 ± 1 °C) and relative humidity (50-70%) before starting the cycle.
The total airborne disinfection contact time (ADC) is recorded, starting from the first release of the product to the point where the carriers are retrieved or to the point where the deactivation (aeration) starts, if necessary.
The carriers are then processed in a recovery liquid and plated out onto appropriate agar plates. The results are counted, and the achieved efficacy expressed in LOG reduction.
Distribution Test
The test follows the same methodology of the efficacy test with the aim of assessing the efficacy of the system throughout the chamber. For this reason, a total of 8 carriers are placed in the four corners of the room and specified heights and distances from wall/floor/ceiling. It is performed with only one organism, S. aureus, and must be passed to claim compliance with the EN 17272 standard.
Depending on the area and organism, different LOG reductions are required as detailed in the table below.
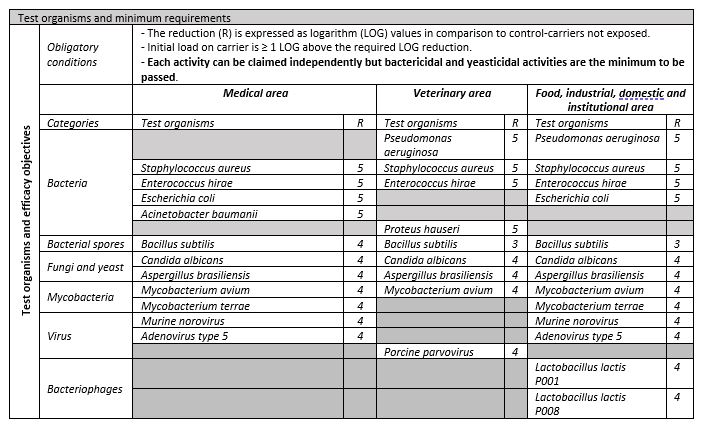
Test Labs have implemented the EN 17272 standard in our laboratory and have gained UKAS accreditation under the ISO 17025 scope.
Get It Done, With Certainty.
Contact us about your testing requirements, we aim to respond the same day.
Get resources & industry updates direct to your inbox
We’ll email you 1-2 times a week at the maximum and never share your information


