Biological Evaluation Plan: Everything You Need to Know
Article Summary
Part of the risk management process, a BEP (Biological Evaluation Plan) is an essential task for manufacturers of medical devices that have direct or indirect contact with patients or users. It's a requirement for compliance with the ISO 10993-1 standard, and lack of BEP documentation is a leading cause of rejection when submitting to notified bodies or competent authorities.Article Contents
What is biological evaluation plan?
Biological evaluation plan is a risk management activity in which manufacturers consider possible biological hazards and risks associated with the clinical use of the device. The process of evaluating the biocompatibility of a device is carried out in accordance with ISO 10993-1 : Biological evaluation of medical devices: Part 1: Evaluation and testing within a risk management process.
The initial step in performing this assessment is to plan biological evaluation activities and document these in a Biological Evaluation Plan. The plan evaluates the risk from available data including device configuration, material composition, manufacturing processes and processing agents, intended use, any testing information and clinical history. This assessment identifies gaps in the available data which further investigation and testing may be required to provide sufficient information on the toxicological risk.
Biological Evaluation Plan evaluates the risk from available data including device configuration, material composition, manufacturing processes and processing agents, intended use, any testing information and clinical history.

Why do Manufacturers need a Biological Evaluation Plan?
Producing a structured plan is a requirement to prove compliance to the ISO 10993-1 standard.
ISO 10993-1 states that “biological evaluation of any material or medical device intended for use in humans shall form part of a structured biological evaluation plan…”.
Additionally, the FDA specific guidance document Use of International Standard ISO 10993-1 “Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process” states “a plan should be developed to address the knowledge gaps either by biocompatibility testing or other evaluations…”
One of the leading causes in rejection of device submission to notified bodies or competent authorities is the lack of biological evaluation documentation.
While many manufacturers will have completed testing on their devices, they will not always have completed a biological evaluation plan or biological evaluation report as required by the ISO 10993 series.
What set the requirements of the Biological Evaluation Plan?
Only devices that have direct or indirect contact with either a patient or the device user need to be assessed.
- Direct contact – devices touching patient/user skin, contacting blood pathway or internal tissues. E.g. surgical gloves which contact the skin of the surgeon (user) and internal tissues/blood pathways of the patient.
- Indirect contact – will contact the user/patient indirectly through either contact with another device or contact with products administered into the body. E.g. device cleaning agents and infusion sets respectively.
Devices are categorised by their contact type and contact duration. Devices which contact internal tissue for a long-term period pose a much higher biological risk than devices that only contact intact skin for short periods of time.
Once a device’s contact duration and contact type have been determined, the biological endpoints for consideration can be determined from the table details in Annex A of ISO 10993-1. The FDA guidance document Use of International Standard ISO 10993-1 “Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process” includes additional endpoints for consideration in Table A.1 and should be referred to when submitting for FDA approval. The tables should not be considered a checklist for testing. The device may require more or fewer endpoint than indicated, so device specific guidance should be consulted where appliable.
ISO 10993-1 emphasises the need for physical and chemical characterisation in determining endpoint compliance. Devices manufactured from well characterised materials, or which have a long history of safe use in already marketed devices may not require additional testing to be performed, if duly justified.
The diagram below from ISO 10993-1 shows the decision process that should be followed when determining if there is sufficient toxicological data available prior to considering additional testing. To show compliance with the standard “Biological evaluation should planned, carried out and documented by knowledgeable and experienced professionals” which ensures that suitable judgement is made when determining if sufficient toxicological data is available. This process benefits manufacturers by ensuing no unnecessary testing is performed.
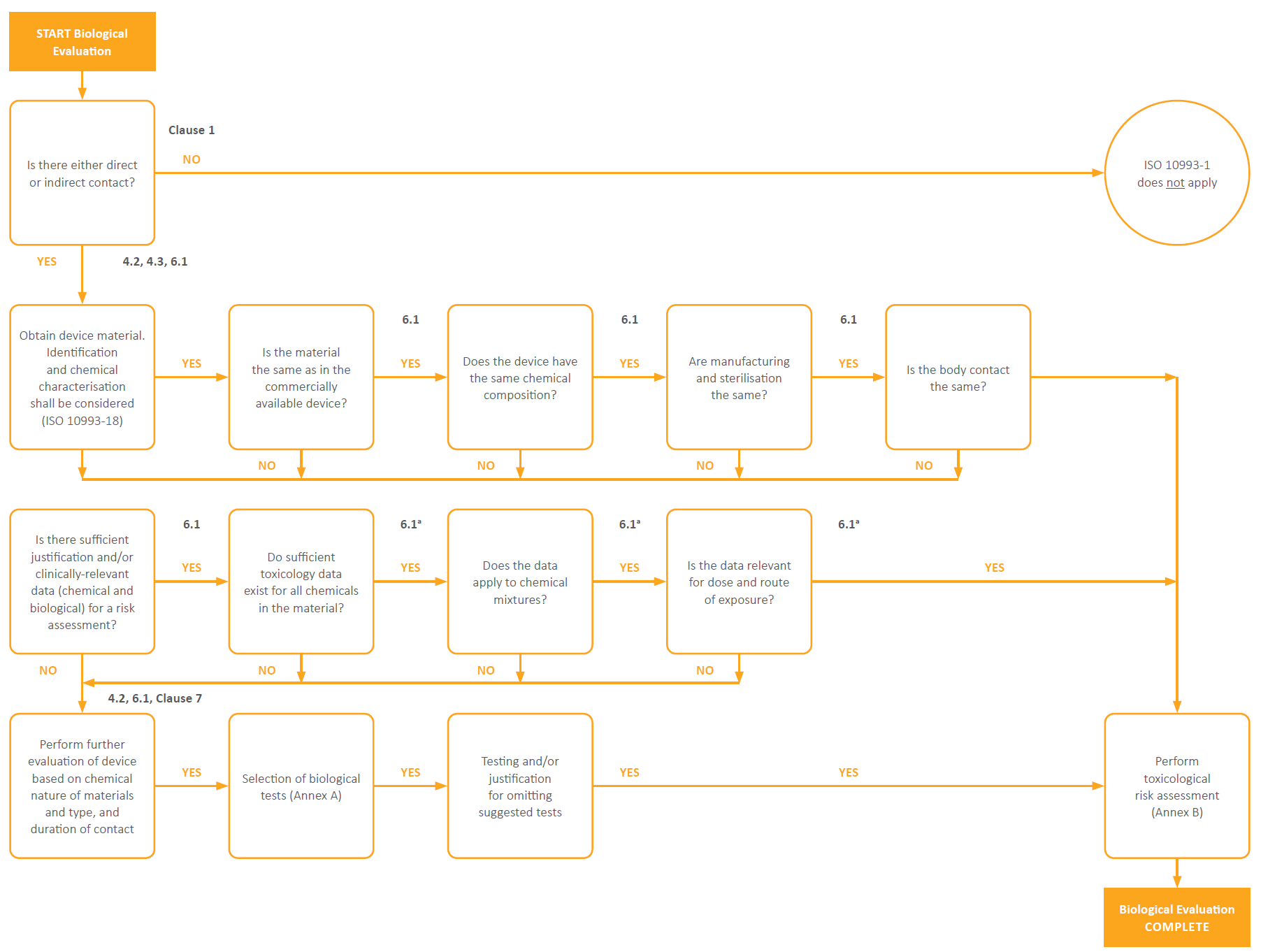
Figure 1 – Summary of the systematic approach to a biological evaluation of medical devices as part of a risk management process.
What information is reviewed in a Biological Evaluation Plan?
The following information is gathered and assessed when performing biological risk gap analysis:
- Device intended use
- Device configuration and physical characteristics
- Material constituents
- Manufacturing processes
- Manufacturing additives and processing agents which may result in residues
- Sterilization processes
- Packaging materials
- Leachable substances and degradation products
- Available toxicological data and biological safety data on materials
- Systemic and local effects of implantable devices
- Historical clinical data
It is important that manufacturers start this process early. Identifying potential risks from materials during the design and development stages can mean avoiding additional testing costs down the line and allows for selection of suppliers who are willing to provide the necessary data on their materials for evaluation.
Get It Done, With Certainty.
For further information on Biological Evaluation Plan for your medical device, please contact our team and we would be happy to help you on your biocompatibility journey.
Get resources & industry updates direct to your inbox
We’ll email you 1-2 times a week at the maximum and never share your information


